NUVO provides efficient solutions to making quality Drug Master File (DMF) submissions for entire Europe including the Certificate of Suitability (CEP/COS) submission to EDQM. We guide with complete process and documentation required for the safe filing and review of the active substance master file (ASMF).
Following are the activities and services provided by NUVO in brief :
ASMF and CEP module writing and Submissions services by NUVO:
- ASMF and CEP Due diligence
- ASMF and CEP audit
- ASMF and CEP conversion from Paper/CTD to eCTD
- Module writing for AP and RP of ASMF for Active pharmaceutical ingredient (API), Intermediates and Semi-finished dosage forms.
- Module writing for CEP dossier for Active pharmaceutical ingredient (API)
- All modules Viz. Module 1, Module 2 and Module 3 writing for ASMF and CEP
- ASMF and CEP module writing in CTD, publishing in eCTD and Submission by CESP
- Query response assistance while review when referenced to MAA application
- Assistance in response to request for additional information
- Support and assistance to overcome validation issues if any.
eCTD Publishing services :
- eCTD Compilation, QC Review, Technical Validation and Submission Hosting
- eCTD Lifecycle Management
- Baseline eCTD : Dossier conversion from Paper/CTD/NeeS to eCTD.
- Importing and Cloning of Existing eCTD Applications
- eCTD publishing for Original ASMF for API, Intermediate and formulation.
- eCTD publishing for CEP for API, Intermediate
- Any changes to existing ASMF or CEP revision filing.
- Query response for request for additional information
- Assistance in OCR (Optical Character Recognition), Embed Fonts, Compliant with Regulatory Requirements
- Assistance in setting up PDF attributes
- Assistance in hyperlinking
- Pdf publishing: PDF – Ready for Submission, Bookmarking, Hyperlinking,
ASMF Submission Services:
- We assist in ASMF submission to European health authorities via its submission portal CESP.
- We assist in CEP submission to EDQM
- All the above mentioned eCTD published ASMF in original, along with changes and query response can be submitted by you to US FDA.
Life-cycle management of Active Substance master File (ASMF) and CEP :
- ASMF revisions preparations, eCTD publishing and filing with US FDA
- Query response to support the amendment filing to safe approval
- ASMF updating and its complete revision.
- CEP revisions
- CEP renewals
For more details and understanding on ASMF read below:
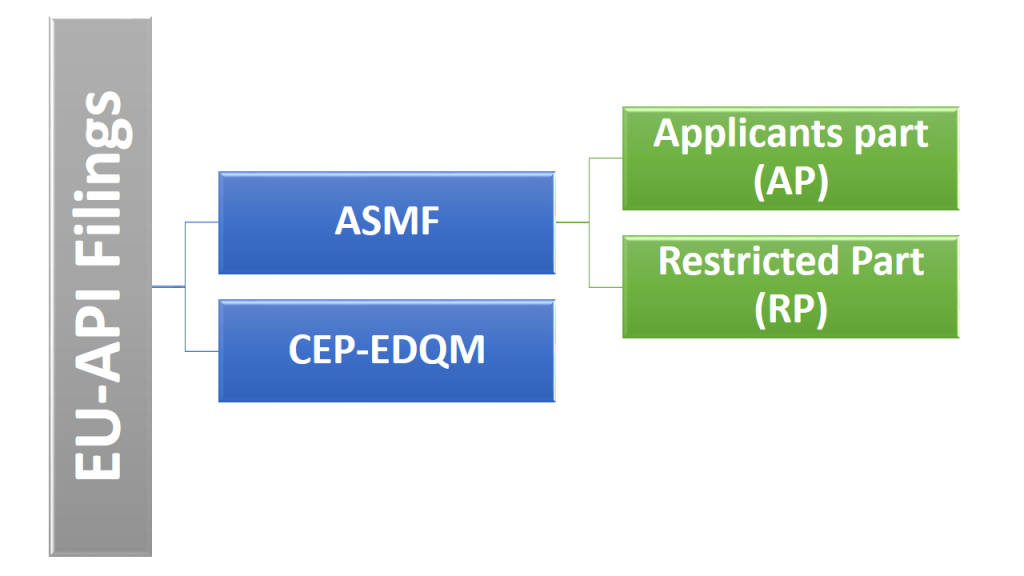
Active Substance Master File (ASMF) or Drug master file (DMF) or CEP is the set of series of information for an Active Pharmaceutical Ingredient (API) or Drug substance brought together in CTD format as per regulatory requirements. ASMF and CEP provide confidential detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of one or more human drugs.
CEP is the dossier submitted to EDQM and undergoes review process for its approval before referencing in the MAA.
ASMF are submissions to European regulatory agencies across Europe in all individual countries used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products. ASMF is never approved nor disapproved, but is reviewed everytime it is referenced in the MAA application unlike CEP is approved.
ASMF constitutes of two parts, Applicants Part (AP) and Restricted Part (RP).
Applicant’s part has the information on chemistry manufacturing and control (CMC) information of API included in it for the customer reference for its inclusion in its Marketing Authorization Application (MAA) Dossier. Which is further submitted to various European agencies through National, Mutual Recognition Procedure (MRP) or Repeat Use Procedure (RUP) and Decentralized Procedure (DCP) procedures.
Restricted Part alsohas the information on chemistry manufacturing and control (CMC) information of API included in it for the regulatory health authority reference for its evaluation against the Dossier submitted in MAA to various European agencies through National, MRP/RUP and DCP procedures.
They:
- Allow parties to reference material without disclosing restricted part of ASMF contents to those parties.
- Are not required by statute or regulation.
- Are neither approved nor disapproved. Instead, euorpean regulatory health authorities reviews the technical contents of ASMF in connection with the review of MAA that reference them (e.g. All MAA filed under Article 10.1, 10.3, 10.a and so on).
NUVO provides efficient technical writing and submission of US DMF for our clients globally.
All documents and data required for the ASMF and CEP submissions are evaluated and aligned as per the international requirements and guidance. We provide customized service for advice and complete project management for filing of following types of dmf :
- ASMF : AP and RP – Drug substance, drug substance intermediate, and materials used in their preparation, or drug product
- CEP dossier writing for all the modules and preparation with eCTD publishing and submission to EDQM
Please contact info@nuvoconsultancy.com with all CEP and ASMFrelated submission questions.